The FDA recently approved a new therapy, Litfulo (ritlecitinib) from Pfizer, Inc., a once-daily oral treatment, for individuals 12 years of age and older with severe alopecia areata.
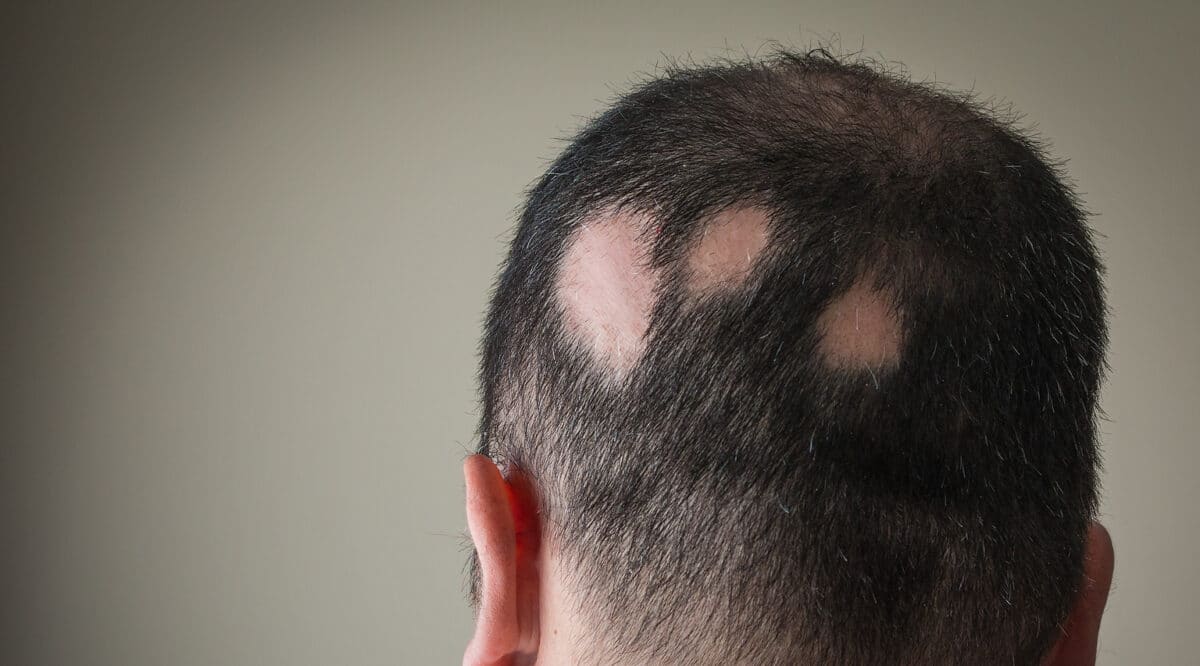
Alopecia areata is an autoimmune disease characterized by patchy or complete hair loss on the scalp, face, or body. It has an underlying immuno-inflammatory pathogenesis and develops when the immune system attacks the body’s hair follicles, causing hair to fall out. This hair loss often occurs on the scalp, but it can also affect eyebrows, eyelashes, facial hair, and other areas of the body. Alopecia totalis (total scalp hair loss) and alopecia universalis (total body hair loss) are types of alopecia areata.
Alopecia Areata impacts nearly 7 million people in the U.S. and approximately 147 million people globally and can affect people of any age, gender, race, or ethnicity and can cause considerable burden beyond hair loss. Nearly 20% of people with alopecia areata are diagnosed before the age of 18.
The therapy was approved with a Boxed Warning that includes Serious Infections, Mortality, Malignancy, Major Adverse Cardiovascular Events (Mace), and Thrombosis.
Pfizer announced that a full year supply of Litfulo carries a list price of $49,000, similar to other specialty dermatologic treatments.
Distribution and logistics for the therapy were not announced but it is highly likely it will launch through specialty pharmacy limited distribution.
CLICK HERE to access prescribing information
——————————————————————————————–
FDA Approves Pfizer’s LITFULO for Adults and Adolescents with Severe Alopecia Areata
LITFULO is the first and only treatment for severe alopecia areata approved for patients
June 23, 2023 — NEW YORK–(BUSINESS WIRE)– Pfizer Inc. (NYSE: PFE) announced today that the U.S. Food and Drug Administration (FDA) has approved LITFULO (ritlecitinib), a once-daily oral treatment, for individuals 12 years of age and older with severe alopecia areata. The approved recommended dose for LITFULO is 50 mg. It is the first and only treatment approved by the FDA for adolescents (12+) with severe alopecia areata.
“While patients may start to develop symptoms of alopecia areata at any age, most people start showing signs in their teens, twenties, or thirties,” said Dr. Brittany Craiglow, Associate Professor Adjunct – Dermatology at Yale School of Medicine. “LITFULO is a particularly important treatment option for younger patients with substantial hair loss, who often struggle with such a visible disease.”
LITFULO is a kinase inhibitor which inhibits Janus kinase 3 (JAK3) and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) family of kinases.
“LITFULO is an important treatment advancement for alopecia areata, an autoimmune disease that previously had no FDA-approved options for adolescents and limited options available for adults,” said Angela Hwang, Chief Commercial Officer, President, Global Biopharmaceuticals Business, Pfizer. “With today’s approval, adolescents and adults who struggle with substantial hair loss have an opportunity to achieve significant scalp hair regrowth.”
The FDA approval was based on results of clinical trials in alopecia areata. The ALLEGRO Phase 2b/3 trial, which enrolled 718 patients with 50% or more scalp hair loss as measured by the Severity of Alopecia Tool (SALT), evaluated the efficacy and safety of LITFULO at 118 sites in 18 countries. In this pivotal study, 23% of patients treated with LITFULO 50 mg had 80% or more scalp hair coverage (SALT≤20) after six months compared to 1.6% with placebo. The efficacy and safety of LITFULO were consistent between adolescents (12 through 17 years of age) and adults (18 years of age and older). The most common adverse events (AEs) reported in at least 4% of patients with LITFULO include headache (10.8%), diarrhea (10%), acne (6.2%), rash (5.4%), and urticaria (4.6%). Full results from the ALLEGRO Phase 2b/3 study were published by The Lancet in April 2023.
“People living with alopecia areata are often misunderstood, and their experience is frequently trivialized as ‘just hair.’ However, it is a serious autoimmune disease that can have considerable negative impact beyond the physical symptoms,” said Nicole Friedland, President and Chief Executive Officer of the National Alopecia Areata Foundation (NAAF). “We believe the approval of LITFULO is a significant advancement for the treatment of alopecia areata, particularly for teens. It’s exciting to see more FDA-approved treatments becoming available for this community.”
View the full Prescribing Information. If it is not currently available via this link, it will be visible as soon as possible as we work to finalize the document. Please check back for the full information shortly.