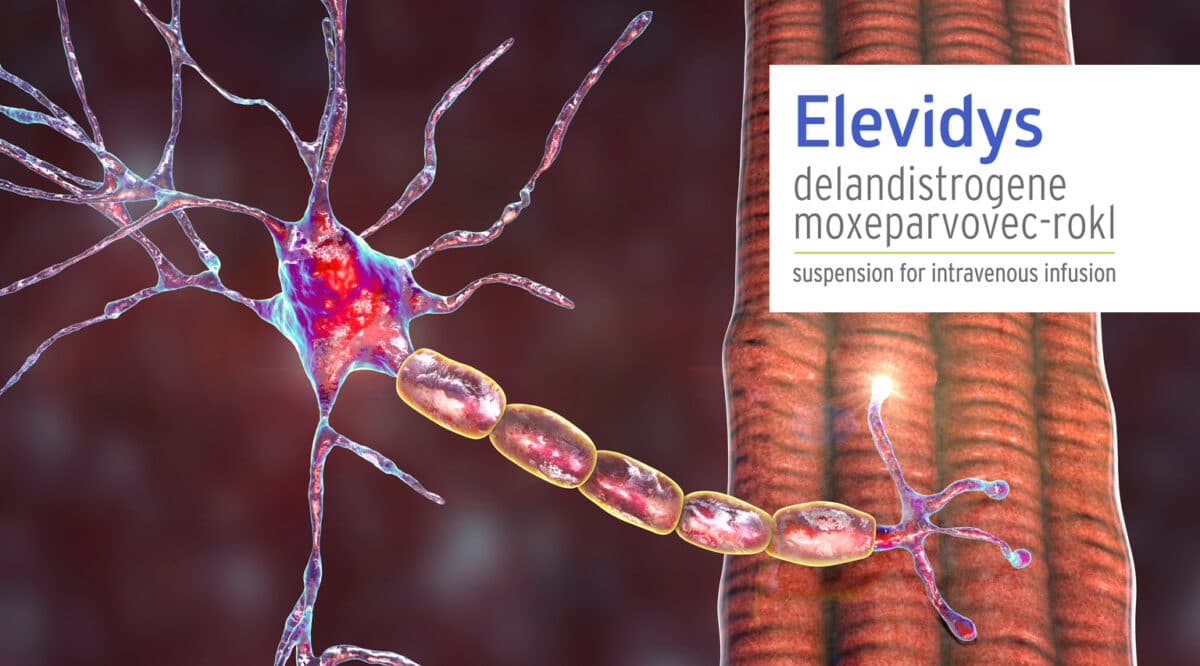
There was a big development for DMA patients last June with the FDA approval of Elevidys (delandistrogene moxeparvovec-rokl) from Sarepta Therapeutics. Elevidys was the first and only gene therapy approved for Duchenne Muscular Dystrophy (DMD). But there was were two hitches in the giddyap…it was approved only for patients with a confirmed mutation in the DMD gene, and, its $3.2 million cost makes access a real obstacle.
We missed covering the more recent approval of another therapy for DMD that is not a gene therapy….. but one that has broad application in the category. The FDA approved Agamree (vamorolone) from Santhera Pharmaceuticals, a novel corticosteroid with favorable side effects. It is provided in an oral suspension form.
It is estimated that between 11,000 and 13,000 patients in the U.S. are affected by DMD, with approximately 70% of patients currently receiving concomitant corticosteroid treatment. DMD, is the most common form of muscular dystrophy, is a rare and life-threatening neuromuscular disorder characterized by progressive muscle dysfunction, ultimately leading to loss of ambulation, respiratory failure, and death.
Click Here for prescribing information
Catalyst holds the exclusive north American license to commercialize Agamree.
Pricing for Agamree is not nearly as costly as the big ticket gene therapy at under $10,000 per year for most patients based on weight. There has been no update on limited distribution.
—————————————————————–