………. Catching up on FDA approvals
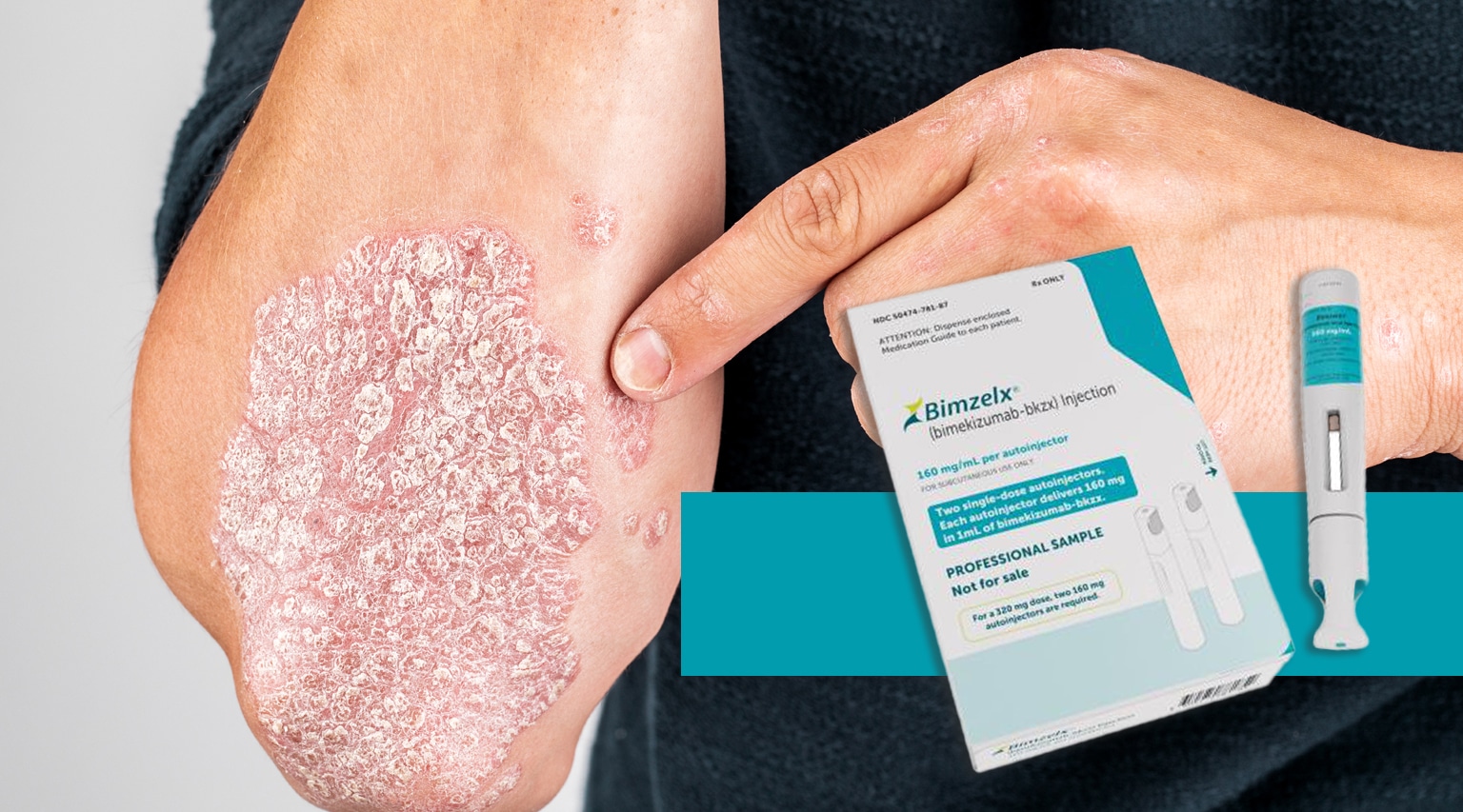
The FDA approved a new therapy at the tail end of last year (it was November and, yes, we are trying to get caught up with the flood of approvals at the end of 2023)….. anyways…. the new therapy is Bimzelx (bimekizumab-bkzx) from UCB approved for the treatment of adults with moderate to severe plaque psoriasis.
Bimzelx is the first and only approved psoriasis treatment designed to selectively inhibit two key cytokines driving inflammatory processes – interleukin 17A (IL-17A) and interleukin 17F (IL-17F).
Click Here for prescribing information
UCB is offering Bimzelxr as an autoinjector or pre-filled syringe at a list price of $7,200 per syringe. The recommended dosage is 320 mg (given as two subcutaneous injections of 160 mg each) at weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. That translates into 10 cycles per year requiring 2 syringes per cycle or 20 syringes which prices out annually at $144,000.
Unlike almost every other specialty manufacturer, UCB has created an “Enhanced Network of Specialty Pharmacies” that provide additional product-specific patient support. Even more noteworthy, UCB is making the therapy available through all other specialty pharmacies….. but without all the ‘extra’ bennies available through the ‘enhanced’ SPs.
CLICK HERE for the ‘Enhanced’ list
———————————————————————————
BIMZELX Approved by the U.S. FDA for the Treatment of Adults with Moderate to Severe Plaque Psoriasis