Announcements for newly approved specialty drugs often state that the product will be available through specialty pharmacy in limited distribution. However, the press releases rarely specify the specialty pharmacy(ies) selected as the designated partner(s).
Here is a basket full of LD deals that have been recently publicly confirmed subsequent to FDA approval.
AcariaHealth to dispense Elevidys
AcariaHealth announced that it has been selected by Sarepta Therapeutics, Inc. as part of the limited distribution network for gene therapy Elevidys (delandistrogene moxeparvovec-rokl), approved for the treatment of ambulatory pediatric patients aged four through five years with Duchenne muscular dystrophy (DMD) with a confirmed mutation in the DMD gene.
Amber Specialty Pharmacy Selected to Dispense Vowst
Amber Specialty Pharmacy has been selected as one of only two pharmacies nationally selected by Aimmune Therapeutics, to support the launch of Vowst indicated to prevent the recurrence of Clostridioides difficile infection (CDI) in individuals 18 years of age and older following antibacterial treatment for recurrent CDI (rCDI).
Orsini Specialty Pharmacy to dispense Vowst
Orsini Specialty Pharmacy announced that Aimmune Therapeutics has selected it as a limited distribution partner for Vowst (fecal microbiota spores, live – brpk) indicated to prevent the recurrence of Clostridioides difficile infection (CDI) in individuals 18 years of age and older following antibacterial treatment for recurrent CDI (rCDI).
Orsini Specialty Pharmacy to dispense Austedo
Orsini Specialty Pharmacy announced that they now has access to Austedo XR (deutetrabenazine) for treating Tardive Dyskinesia (TD) and Chorea associated with Huntington’s Disease (HD).
Orsini pharmacy selected to supply Roctavian
Orsini Specialty Pharmacy has been selected to distribute the new gene therapy Roctavian (valoctocogene roxaparvovec-rvox) for use by individuals with hemophilia A. The one-time treatment from BioMarin Pharmaceutical is the first gene therapy ever approved by the FDA for eligible adults.
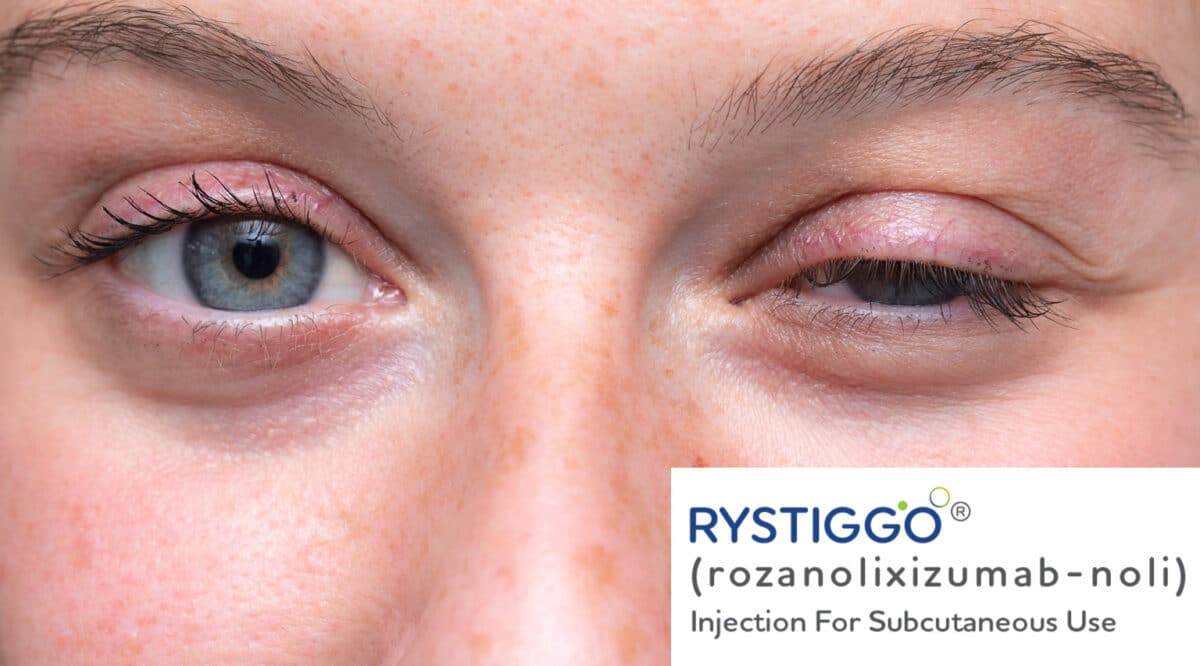
PANTHERx Rare selected to supply Rystiggo
PANTHERx Rare announced that it has been selected by UCB as a limited distribution partner to provide Rystiggo (rozanolixizumab-noli) FDA-approved treatment for both anti- acetylcholine receptor (AChR) anti- muscle-specific tyrosine kinase (MuSK) antibody positive adult patients with generalized myasthenia gravis (gMG).