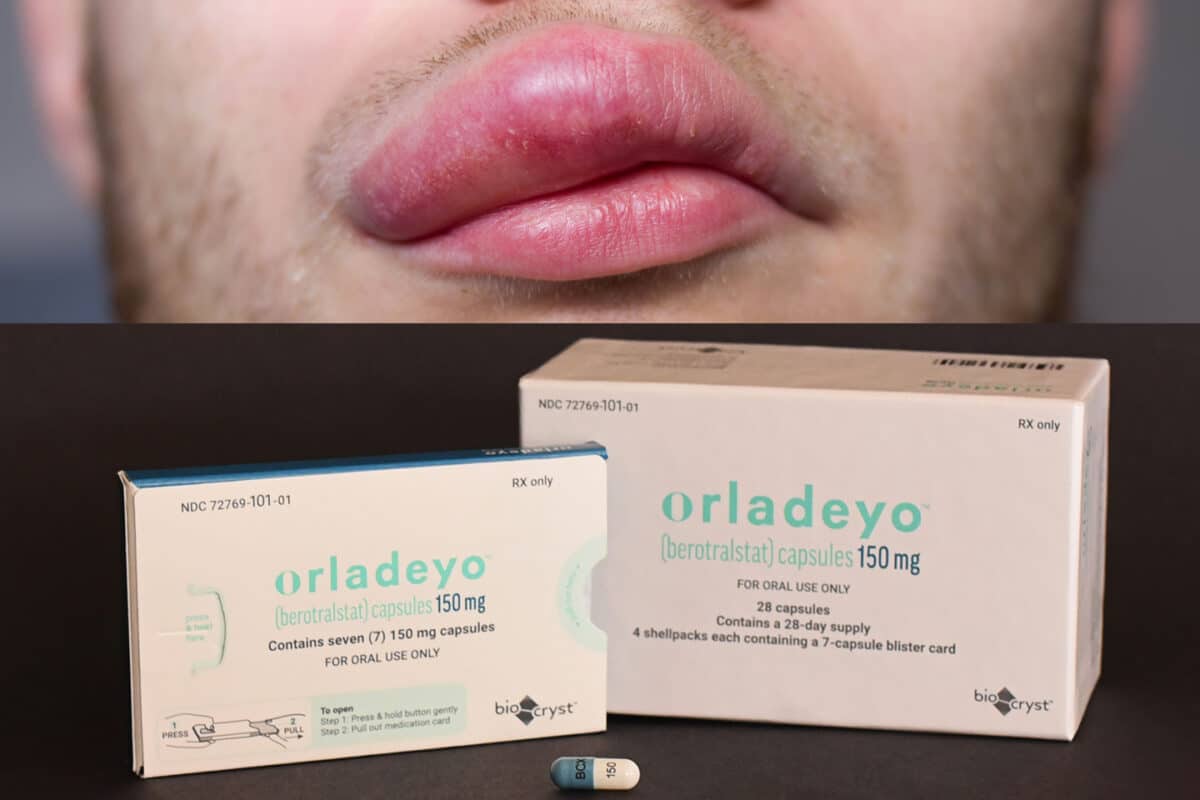
Earlier this month the FDA approved yet another orphan drug, Orladeyo (berotralstat) from BioCryst Pharmaceuticals. It obtained an indication for the prevention of hereditary angioedema (HAE) attacks. Orladeyo is the first oral, non-biologic, once daily treatment for this condition.
HAE is caused by a genetic defect causing a biochemical imbalance that releases fluids outside of the blood vessels into surrounding tissues. Symptoms include swelling in various parts of the body, including the hands, feet, face and airway. Airway swelling can lead to death by asphyxiation. Before therapies became available, the mortality rate for airway obstruction was as high as 30%. HAE defect interferes with a blood protein (called C1 inhibitor) that helps to regulate blood-based systems involved in disease fighting, inflammation and coagulation.
Only 7,500 people are diagnosed and treated for HAE in the U.S.
Rare conditions are usually lucky to have a single novel therapy for patients. HAE is remarkably different as it has eight….. yep, eight brands to choose from. They include Berinert, Cinryze, Firazyr, Haegarda, Kalbitor, Orladeyo, Ruconest, Takhzyro. Oh, and a few days after the approval of Orladeyo the FDA approved a generic version of Firazyr. The more the merrier??
Orladeyo is a relatively late market entry and will compete with these well-established therapies. Leading competitive therapies include Cinryze and Haegarda, and Takhzyro. However, Cinryze requires intravenous (IV) administration, Haegarda is administered via subcutaneous injection every 3 to 4 days and Takhzyro is SC administered every 2 to 4 weeks. Orladeyo has the advantage of being the first oral formulation.
Specific pricing for Orladeyo was just announced. Many analysts believed that the non-biologic Orladeyo could be priced less than the biologic alternatives currently in use. But NO! Biocryst set the wholesale acquisition cost at $485,004 annually, or $37,308 per 28-day pack of either 150-mg or 110-mg capsules. By comparison, the WAC price for Takhzyro is ~$591,000 per year.
Biocryst announced that it has selected Optime Care Inc. as the exclusive ‘rare disease’ specialty pharmacy provider for Orladeyo. Patient shipments from Optime Care are expected to begin by the end of December.
BioCryst Announces FDA Approval of Orladeyo™ (berotralstat), First Oral, Once-daily Therapy to Prevent Attacks in Hereditary Angioedema Patients
RESEARCH TRIANGLE PARK, N.C., Dec. 03, 2020 (GLOBE NEWSWIRE) — BioCryst Pharmaceuticals, Inc. (Nasdaq: BCRX) today announced that the U.S. Food and Drug Administration (FDA) has approved oral, once-daily Orladeyo™ (berotralstat) for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adults and pediatric patients 12 years and older.
“Orladeyo offers people with HAE and their physicians the first orally administered non-steroidal option for preventing HAE attacks and represents an important and welcome step in making more treatment options available to physicians and patients,” said Anthony J. Castaldo, president and chief executive officer of………