…………………….catching up on FDA approvals
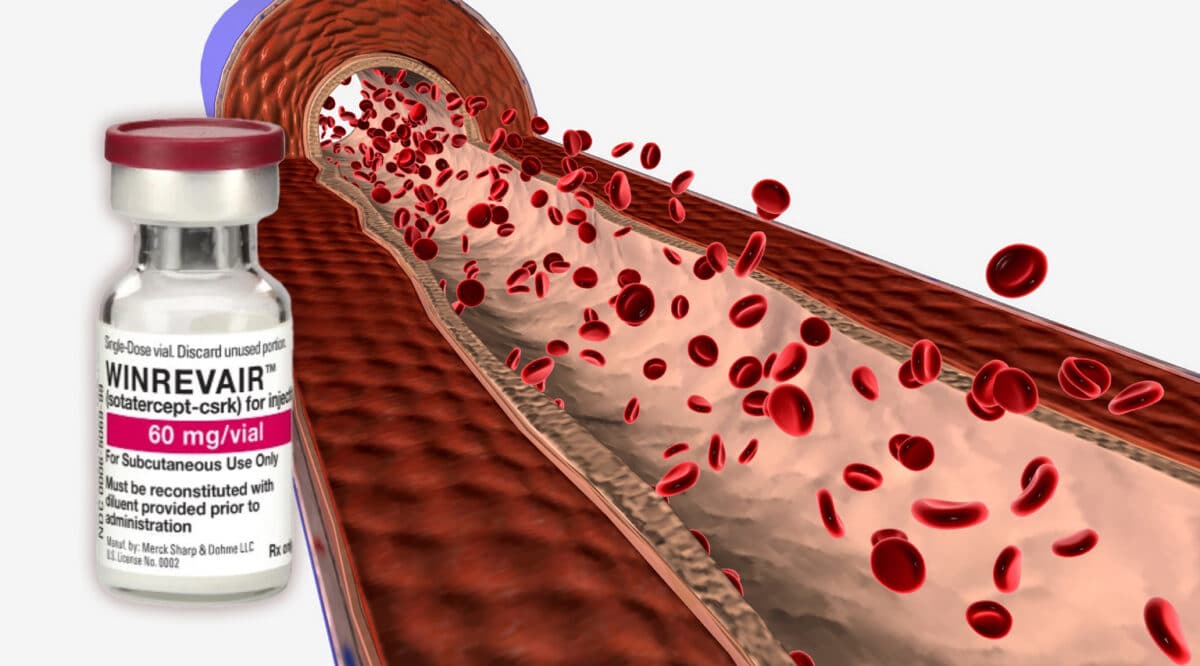
The FDA recently approved a new ORAL therapy, Vafseo (vadadustat) from Akebia Therapeutics, for the treatment of anemia due to chronic kidney disease (CKD) in adults who have been receiving dialysis for at least three months. Vafseo is a once-daily oral hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor that activates the physiologic response to hypoxia to stimulate endogenous production of erythropoietin to manage anemia.
Approximately 500,000 adult patients in the U.S. on dialysis suffer from anemia due to CKD1 which contributes to developing an array of other serious conditions. CKD patients are typically treated for anemia with injectable erythropoiesis-stimulating agents during dialysis. The availability of an oral treatment option is meant to increase and better maintain hemoglobin concentrations within recommended guidelines.
Vafseo was approved with a Black Box warning.
Akembia announced that it is expecting to launch Vafseo in January, 2025. The company also announced that Vafseo will be available for purchase through authorized specialty distributors once the Centers for Medicare & Medicaid Services grants a two-year Transitional Drug Add-on Payment Adjustment (TDAPA).
———————————————————————————–