
Limited Distribution Updates
Announcements for newly approved specialty drugs often state that the product will be available through specialty pharmacy in limited distribution. However, the press releases rarely

Announcements for newly approved specialty drugs often state that the product will be available through specialty pharmacy in limited distribution. However, the press releases rarely

There was a flood of media coverage following the FDA’s announcement that they have approved Florida’s request to import low-cost drugs from Canada. While that may
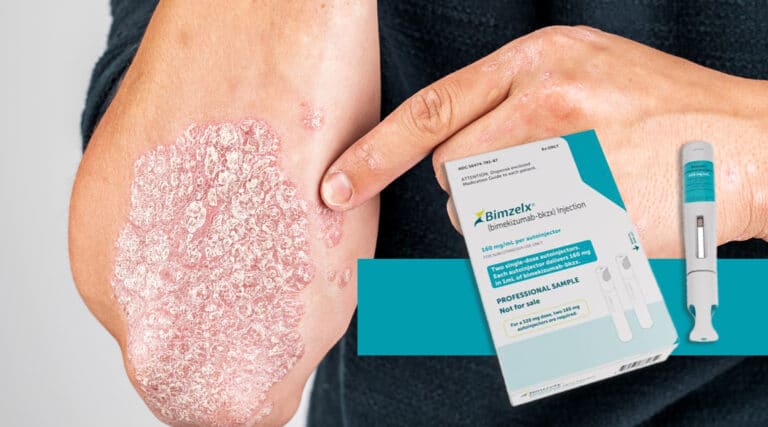
………. Catching up on FDA approvals The FDA approved a new therapy at the tail end of last year (it was November and, yes, we are

Happy New Year….. or to be said otherwise, What’s Happy About It? The article below doesn’t trumpet the benefit of the changes implemented by Congress
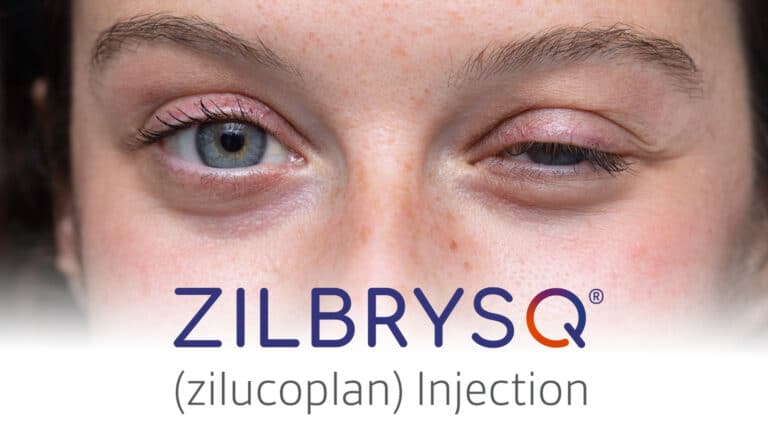
…..catching up on FDA Approvals The FDA has approved a new subcutaneous therapy, Zilbrysq (zilucoplan) from UCB, for the treatment of adults with generalized myasthenia

Specialty pharmacies produce a lot of reporting…… but, the scope of this reporting may be missing the mark in the evolving competitive marketplace. The real challenge
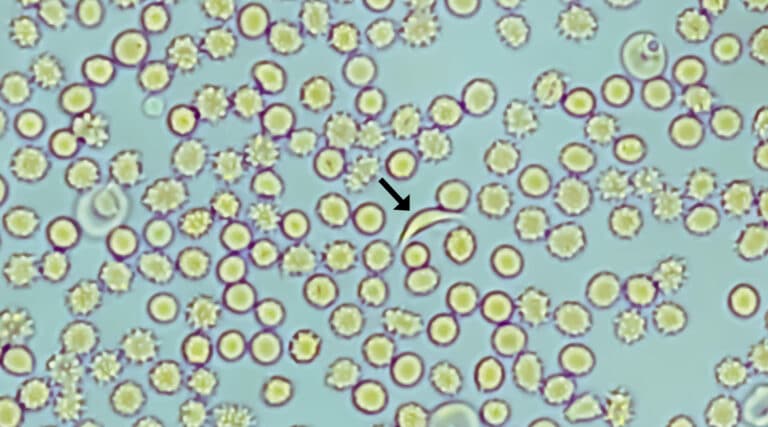
Another milestone has been passed in the advancement of specialty pharmacy therapies. The FDA has approved not one, but two, gene therapies for a major disease,

Announcements for newly approved specialty drugs often state that the product will be available through specialty pharmacy in limited distribution. However, the press releases rarely
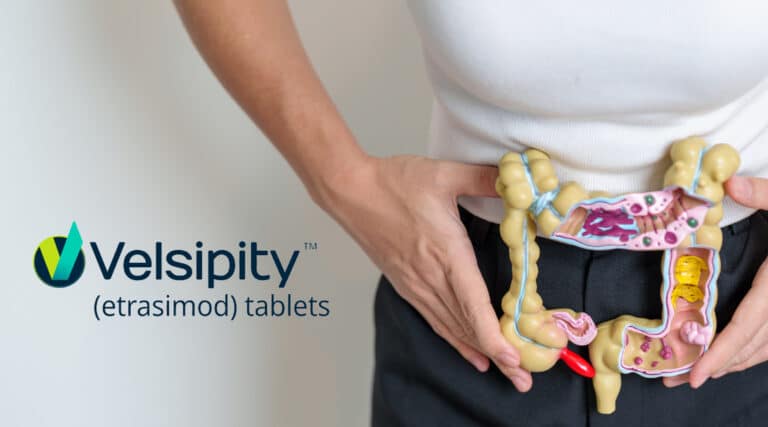
…………..catching up on recent FDA approvals The FDA recently approved a new ORAL therapy, Velsipity (etrasimod) from Pfizer, for adults with moderately to severely active

There has been scant news about any big moves by large national specialty pharmacies this year. Today, however, we read about a move by CarelonRx, the