
AI Meets Sharps Container
Every day we scan hundreds of articles and press releases to find items, events, trends, developments and other flotsam and jetsam related to specialty pharmacy,

Every day we scan hundreds of articles and press releases to find items, events, trends, developments and other flotsam and jetsam related to specialty pharmacy,

Are you familiar with the Preserving Patient Access to Home Infusion Act (PPAHIA)? You probably should be. It has been kicking around the halls of
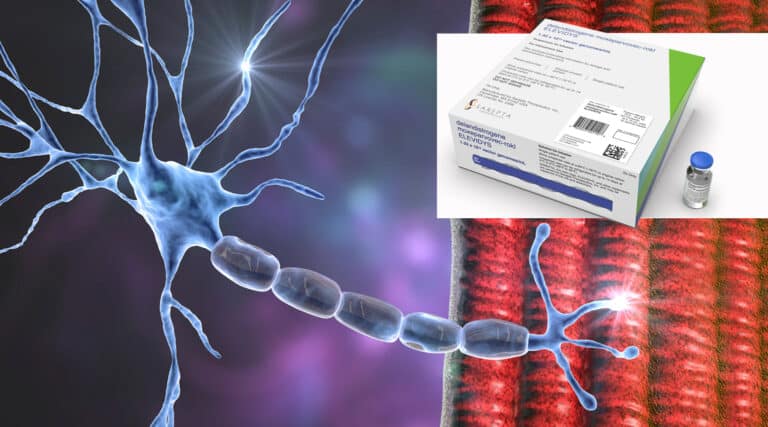
FDA has now approved four gene therapies within the past ninety days. That’s got to be a record! The FDA recently approved a new infused

Not all specialty pharmacies operate a compounding line of business….. but there are a number of SPs that do. If your pharmacy is into compounding, then you
Another sonic boom could be heard (well….. at least by dogs) recently when the FDA approved yet another gene therapy! This time the news was truly
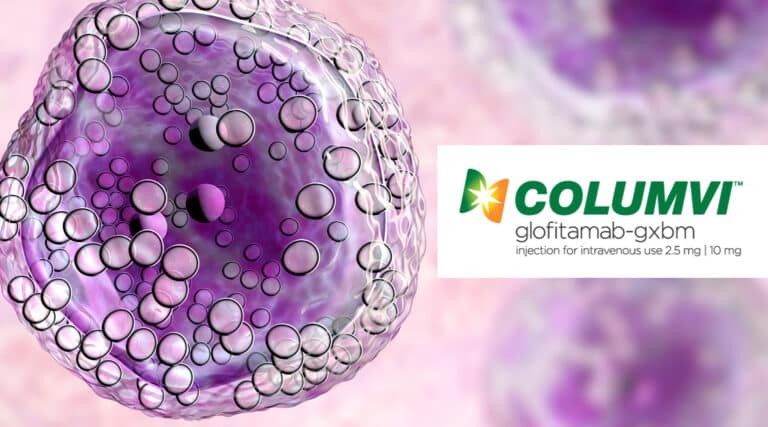
The FDA recently approved a new infused therapy, Columvi (glofitamab-gxbm) from Genentech, for the treatment of adult patients with relapsed or refractory (R/R) diffuse large

A boatload of biosimilars are now sitting in the approval circle since the first one was approved in early 2015. Copies of just four market

Lots of angst between the FEDA and Pharma over drug pricing controls and price negotiations. There will be lobbying a’plenty by Pharma to mitigate what is already

The FDA recently approved a new extended release oral-suspension therapy, Lumryz (sodium oxybate) from Avadel Pharmaceuticals, for the treatment of cataplexy or excessive daytime sleepiness

And the beat goes on…….. We’ve covered the market moves of both CVS and Walgreens as they transition from a pharmacy centric business model to
We use cookies to improve your experience on our site. By using our site, you consent to cookies.
Manage your cookie preferences below:
Essential cookies enable basic functions and are necessary for the proper function of the website.
Statistics cookies collect information anonymously. This information helps us understand how visitors use our website.
Marketing cookies are used to follow visitors to websites. The intention is to show ads that are relevant and engaging to the individual user.
You can find more information about our Cookie Policy and Privacy Policy.