
FDA Approves Oral Tx for AML – Vanflyta
Catching up on recent specialty approvals———— The FDA recently approved a new ORAL, kinase inhibitor therapy, Vanflyta (quizartinib) from Daiichi Sankyo Company, Ltd., in combination

Catching up on recent specialty approvals———— The FDA recently approved a new ORAL, kinase inhibitor therapy, Vanflyta (quizartinib) from Daiichi Sankyo Company, Ltd., in combination

Earlier this week we sent a Report on biosimilar utilization and trends. Today we follow up with some stats from another credible source that add a

A recently released study on payor practices related to biosimilars was detailed in an article published by the Center for Biosimilars….. and some of the
We’ve written recently on legislation grinding its way through Congress targeting the PBM industry and its various business practices that industry stakeholders say are unfair

If you’ve been waiting anxiously (must ask why?) for yet another human growth hormone to be approved….. then wait no longer. The FDA recently approved

The outcome of the FTC review of PBM practices and its report on needed corrections may be some of the biggest news items this year. As
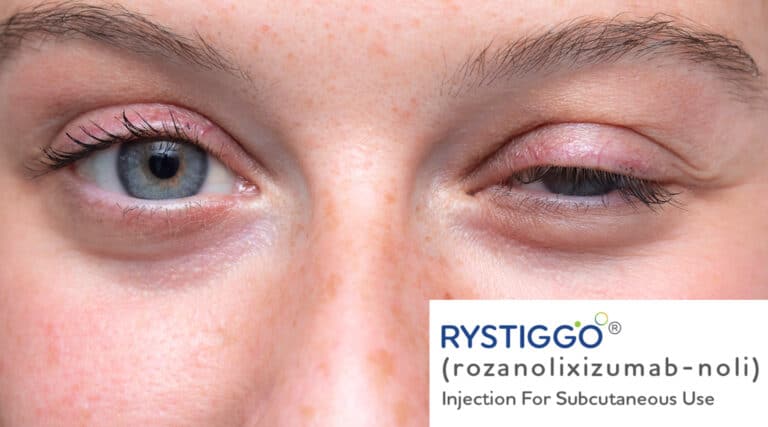
The FDA recently approved a new therapy, Rystiggo (rozanolixizumab-Noli) from UCB, for the Treatment of adults with Generalized Myasthenia Gravis (gMG) in adult patients who are

The year was 2019…… and that BUZZ you were hearing was all about drone deliveries of everything from pizza to prescriptions. But by 2020…. mostly crickets! After that warm
If you haven’t added the term ‘blockchain’ to your vocabulary, then it is high time that you do…. starting right now. Blockchain is a system of
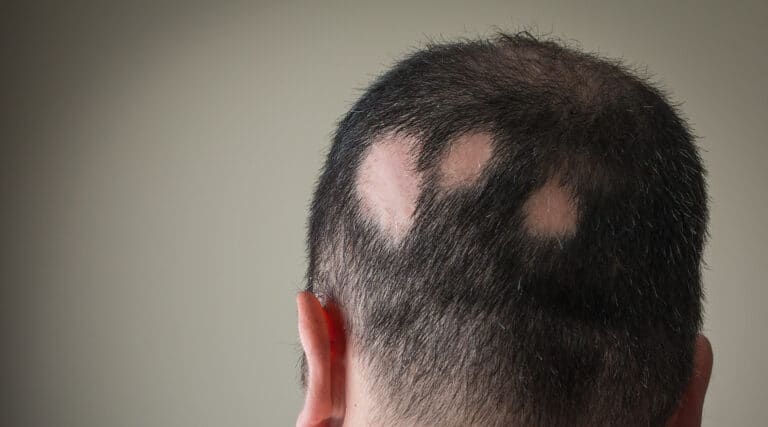
The FDA recently approved a new therapy, Litfulo (ritlecitinib) from Pfizer, Inc., a once-daily oral treatment, for individuals 12 years of age and older with
We use cookies to improve your experience on our site. By using our site, you consent to cookies.
Manage your cookie preferences below:
Essential cookies enable basic functions and are necessary for the proper function of the website.
Statistics cookies collect information anonymously. This information helps us understand how visitors use our website.
Marketing cookies are used to follow visitors to websites. The intention is to show ads that are relevant and engaging to the individual user.
You can find more information about our Cookie Policy and Privacy Policy.