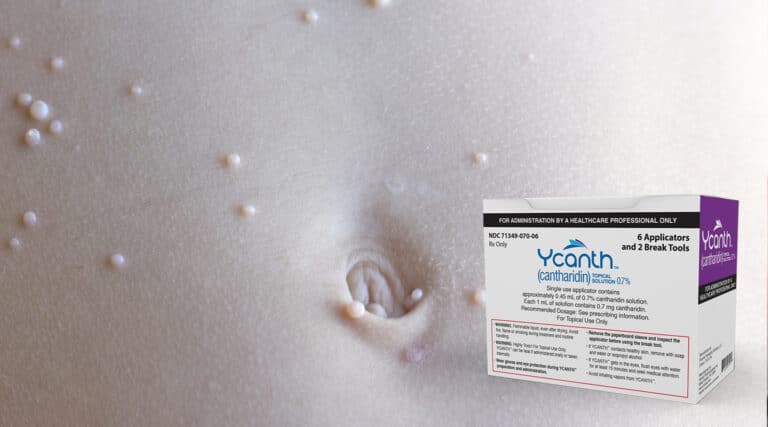
FDA Approves Topical for Viral Skin Condition – Ycanth
Catching up on recent FDA approvals The FDA approved a specialty therapy…. with a difference….. it is a topical ointment. The approval for the new therapy,

Catching up on recent FDA approvals The FDA approved a specialty therapy…. with a difference….. it is a topical ointment. The approval for the new therapy,

Continuing our deep dive into biosimilars It may well take the wisdom of Solomon to fully appreciate the logic of the recently released guidance (or
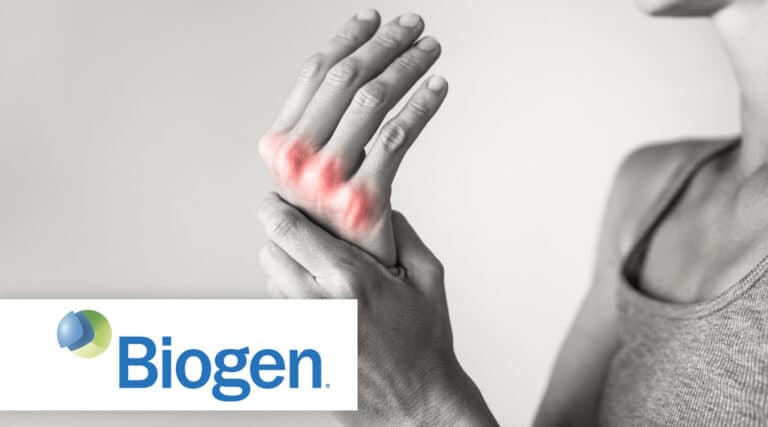
Continuing our focus on Biosimilars The FDA recently approved a new infused biosimilar, Tofidence (tocilizumab-bavi) from Biogen, for the treatment of moderately to severely active

Continuing Biosimilar Week Recently the FDA approved a new biosimilar, Tyruko (natalizumab-sztn) from Sandoz Inc., with indications for adults with relapsing forms of multiple sclerosis,

We are going to hear a lot more about biosimilars in coming days. The leader board now shows that 44 biosimilars have been approved to date…. even

Ok….. here’s something innovative in the wacky world of specialty pharmacy! CVS has launched a new subsidiary called Cordavis. The new entity “will work directly with
The article below was published some weeks ago, but its insight is very timely. It is a simple story….. one in which the Pharma industry is

Catching up on recent FDA approvals. The FDA recently approved a new oral therapy, Sohonos (palovarotene) from Ipsen Biopharmaceuticals, for reduction in the volume of
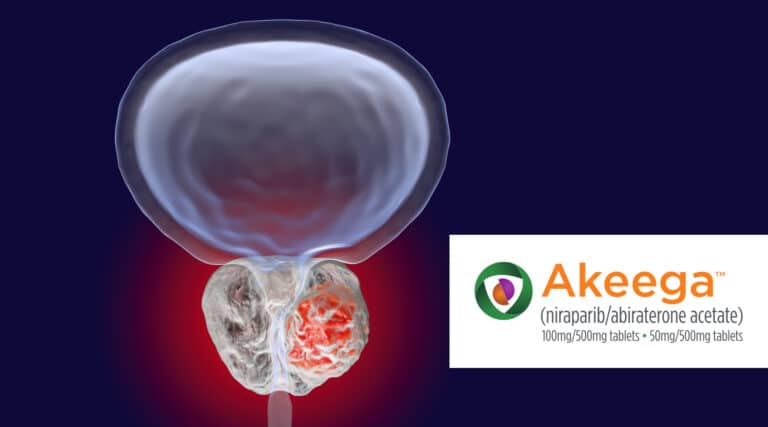
Catching up on recent approvals…….. The FDA recently approved a new oral therapy, Akeega (niraparib and abiraterone acetate) from Janssen Pharmaceutical, the first-and-only dual action

Announcements for newly approved specialty drugs often state that the product will be available through specialty pharmacy in limited distribution. However, the press releases rarely
We use cookies to improve your experience on our site. By using our site, you consent to cookies.
Manage your cookie preferences below:
Essential cookies enable basic functions and are necessary for the proper function of the website.
Statistics cookies collect information anonymously. This information helps us understand how visitors use our website.
Marketing cookies are used to follow visitors to websites. The intention is to show ads that are relevant and engaging to the individual user.
You can find more information about our Cookie Policy and Privacy Policy.