Catching up on recent FDA approvals
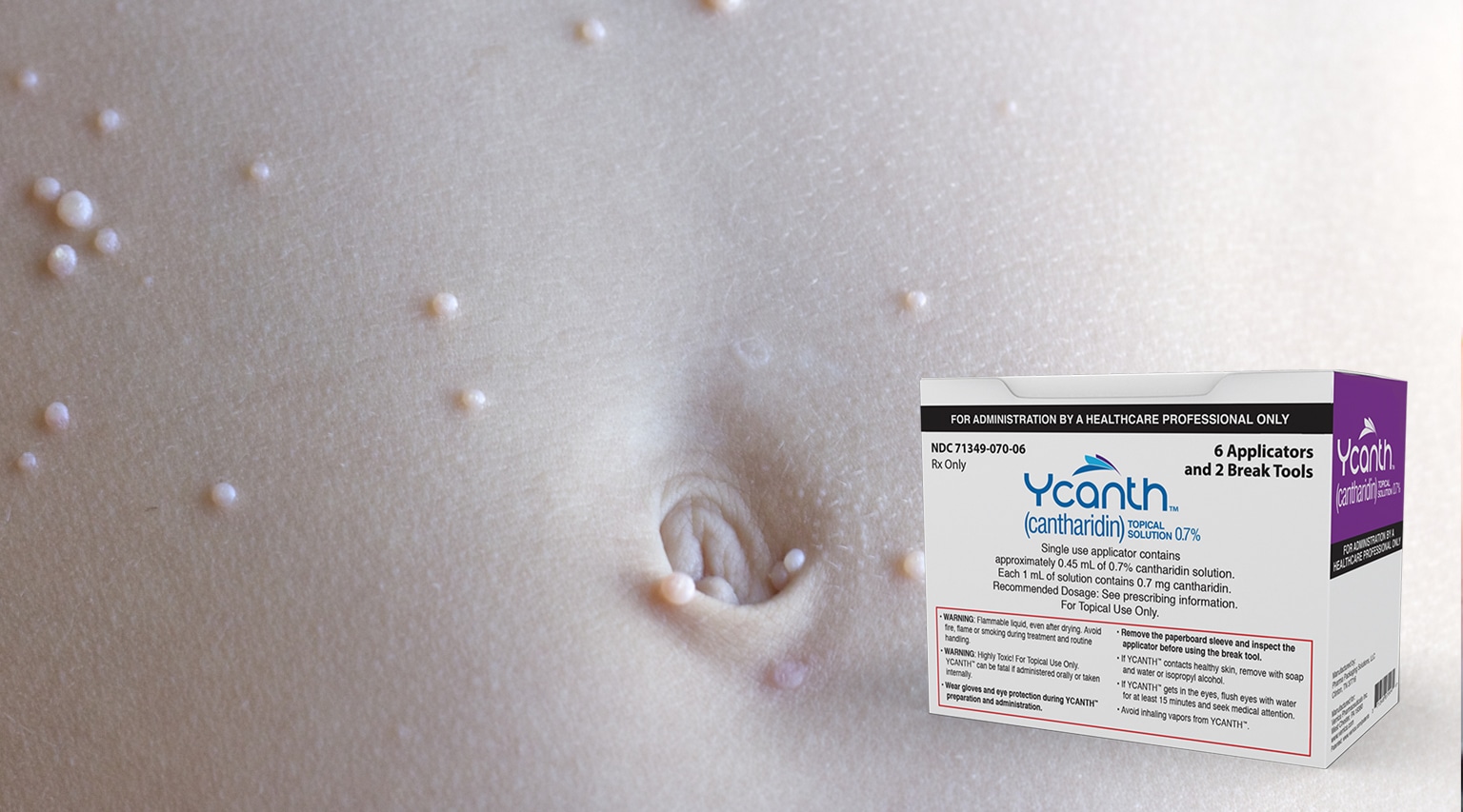
The FDA approved a specialty therapy…. with a difference….. it is a topical ointment. The approval for the new therapy, Ycanth (cantharidin) from Verrica Pharmaceuticals, is indicated for Molluscum Contagiosum in adult and pediatric patients 2 years of age and older. Molluscum Contagiosum is a viral skin infection that cause itchy skin reactions in almost any area of the body. The virus is transmitted by skin-to-skin contact and is most common among children under 10.
Since it is a topical, the first assumption was that specialty pharmacies would be able to dispense this new therapy, however, that’s not the case. It must be administered only by health care providers every 3 weeks as needed. That may be related to one of the serious side effects for Ycanth….. loss of the outer layer of skin at the application site.
The label also includes an unusual patient instruction….. Ycanth is flammable, even after drying. Patients should avoid fire, flame, or smoking near lesion(s) during treatment and after application until removed.
Cost of Ycanth will depend on the number of lesions.
A single carton of 12 applicators is priced at $8500 according to GoodRx.