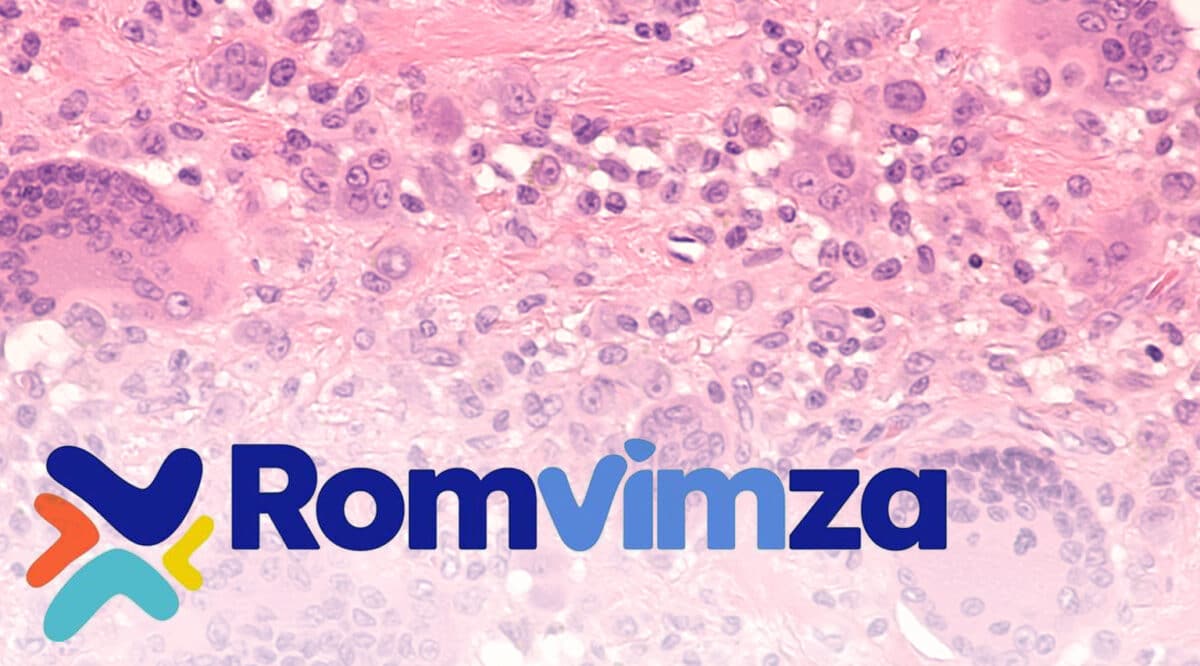
The FDA recently approved a new ORAL therapy, Romvimza (vimseltinib) from Deciphera Pharmaceuticals, LLC, indicated for adult patients with symptomatic tenosynovial giant cell tumor (TGCT) for which surgical resection will potentially cause worsening functional limitation or severe morbidity.
Tenosynovial giant cell tumor (PVNS) or giant cell tumor of the tendon sheath (GCT-TS), is primarily treated through surgical resection. Treatment with TGCT typically involves direct removal of the tumor nodule. Treatment for diffused-type TGCT (DTGCT) with larger tumors affecting major joints, includes total synovectomy, joint replacement, or, in extremely rare cases, or amputation.
The highest prevalence of TGCT accounts for approximately 45% of total cases. Giant cell tumors can occur in the knee, ankle, hip, and other areas. The article cites that diffuse TGCT was most frequently observed in the knee, with an estimated 30,000 cases in the US in 2023.
Pricing details for Romvimza have not yet been announced.
Distribution details have not yet been announced by the company.
CLIC HERE to access prescribing information
——————————————————————————————–