Announcements for newly approved specialty drugs often state that the product will be available through specialty pharmacy in limited distribution. However, the press releases rarely specify the specialty pharmacy(ies) selected as the designated partner(s).
Here are seven LD deals that have been publicly confirmed subsequent to their approvals.
Biologics to Dispense Vanflyta
Biologics by McKesson was selected by Daiichi Sankyo, Inc., as a specialty pharmacy provider for Vanflyta (quizartinib). Vanflyta is indicated for use in combination with standard cytarabine and anthracycline induction and cytarabine consolidation, and as maintenance monotherapy following consolidation chemotherapy, for the treatment of adult patients with newly diagnosed acute myeloid leukemia (AML) that is FLT3-ITD positive as detected by an FDA-approved test.
Orsini Specialty Exclusive Distributor for Veopoz
Orsini Specialty Pharmacy announced that Regeneron Pharmaceuticals, Inc. has chosen Orsini to be the exclusive specialty pharmacy partner for Veopoz (pozelimab-bbfg). A monoclonal antibody, Veopoz is the first and only treatment for those living with CHAPLE disease, an ultra-rare and life-threatening hereditary disease.
Orsini Specialty to Dispense Pombiliti / Opfolda
Orsini Specialty Pharmacy has been selected by Amicus Therapeutics to dispense Pombiliti and Opfolda, a two-component treatment approved for certain patients with late-onset Pompe disease. Pombiliti is a hydrolytic lysosomal glycogen-specific enzyme indicated, in combination with Opfolda, an enzyme stabilizer, for the treatment of adult patients with late-onset Pompe disease (lysosomal acid alpha-glucosidase [GAA] deficiency).
Nufactor Exclusive Specialty Pharmacy for Ycanth
Nufactor, Inc., a subsidiary of FFF Enterprises Inc., announced that it has partnered with Verrica Pharmaceuticals to be the exclusive specialty pharmacy to dispense Ycanth, the first FDA approved treatment of molluscum contagiosum in adult and pediatric patients 2 years of age and older.
Onco360 to Dispense Ojjaara
Onco360 has been selected as a pharmacy partner by GlaxoSmithKline for Ojjaara (momelotinib) a kinase inhibitor indicated for the treatment of intermediate or high-risk myelofibrosis (MF), including primary MF or secondary MF [post-polycythemia vera (PV) and post-essential thrombocythemia (ET)], in adults with anemia.
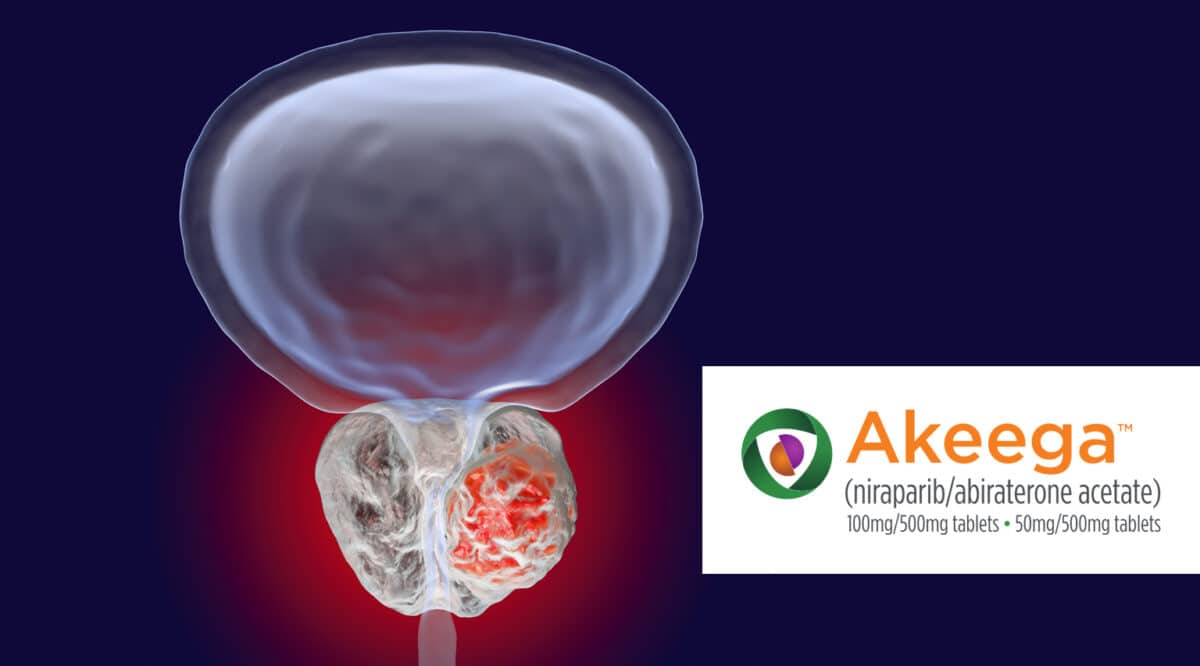
Onco360 to Dispense Akeega
Onco360 has been selected as a pharmacy partner by The Janssen Pharmaceutical Companies of Johnson & Johnson for Akeega (niraparib and abiraterone acetate). Akeega is indicated with prednisone for the treatment of adult patients with deleterious or suspected deleterious BRCA-mutated (BRCAm) metastatic castration-resistant prostate cancer (mCRPC).
CarePartners Pharmacy named exclusive distributor of Yuflyma
CarePartners Pharmacy has been named the exclusive distributor for Yuflyma, a biosimilar to Humira. Yuflyma was approved by the FDA in May, 2023. by Celltrion Healthcare, Celltrion’s distribution unit, said Monday it has signed a deal with US specialty pharmacy chain CarePartners Pharmacy to sell its Humira biosimilar Yuflyma in the US market. Celltrion inked an exclusive contract with CarePartners.