The FDA just approved not ONE, but TWO therapies in the already crowded immunology category.
The two include a new NDA for Cibinqo (abrocitinib) from Pfizer and an expanded indication for well established Rinvoq (upadacitinib).
Let’s look first at Cibinqo —
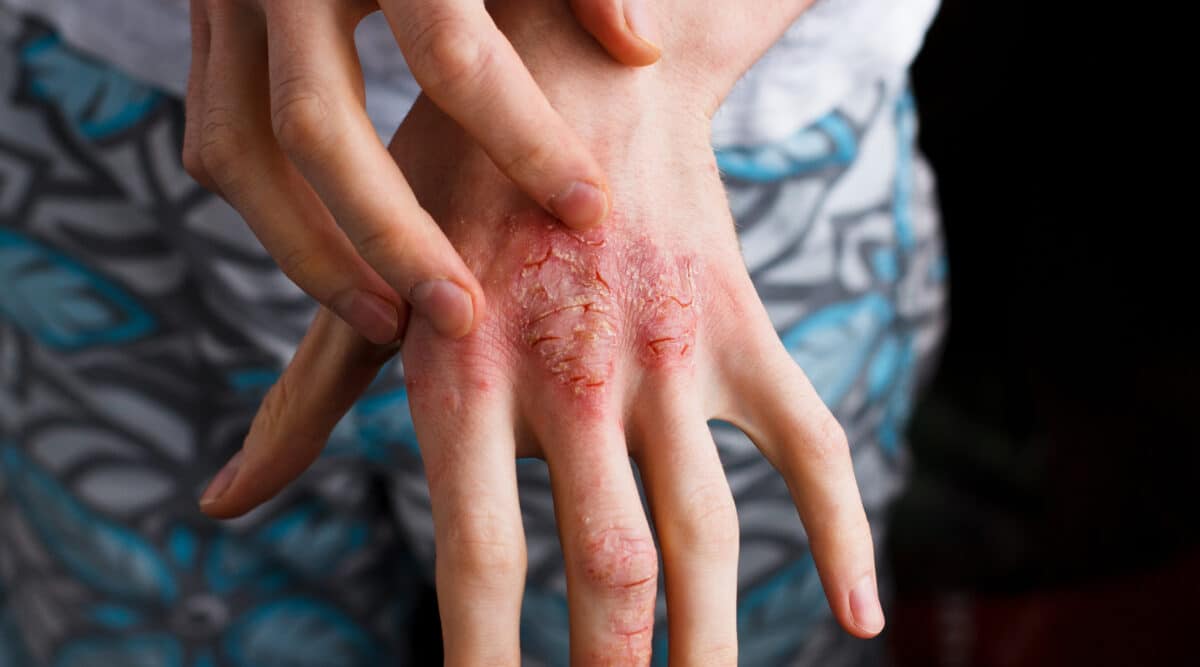
The FDA approved Cibinqo (abrocitinib) from Pfizer, for the treatment of adults living with refractory, moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable. Cibinqo is a once-daily oral. Like its leading competing therapies, it is a Janus kinase 1 (JAK1) inhibitor. Cibinqo is a human monoclonal antibody that inhibits interleukin-13 (IL-13), a cytokine which plays a key role in AD inflammation.
Cibinqo comes with a boxed warning due to observed risk of serious bacterial, fungal, viral and opportunistic infections leading to hospitalization or death, including tuberculosis (TB), a higher rate of lymphomas and lung cancers, cardiovascular death, myocardial infarction, thrombosis, and stroke.
CIBINQO is not approved for use in RA patients.
Pfizer did not announce distribution details. Given the black box warning and the expected cost for Cibinqo, one might expect that it will launch through specialty pharmacy limited distribution. However, Rinvoq is not in limited distribution.
So what about Rinvoq?
Rinvoq was granted an expanded indication for the treatment of adults living with refractory, moderate-to-severe atopic dermatitis (AD). Since its original approval in 2019 for RA. Rinvoq has since established a strong niche in the marketplace. Efficacy between Cibinqo and Rinvoq are comparable. However, Rinvoq’s approval included children 12 and over (vs. Cibinqo’s approval for adults only) although it also carries a black box warning.
Oh, and what about Adbry?
Just last month the FDA approved yet another JAK1 inhibitor therapy, Adbry, also with the AD indication. However, Adbry is an injectable, which may hinder its uptake vs. the newly approved Cibinqo and the newly upgraded Rinvoq….. (both Cibinqo and Rinvoq are orals).
Atopic Dermatitis –
There are an estimated 16.5 million adults in the U.S. living with atopic dermatitis, with approximately 6.6 million categorized as living with moderate-to-severe disease
What about COST?
Pricing for Cibinqo was not announced at the time of approval. Various sources suggest the following anticipated price ranges for the leading competing products.
- Cibinqo — $35,000-$42,000 per year (estimate) for AD
- Adbry — $35,000-$40,000 per year (estimate) for AD
- Olumiant — $24,400-$33,300 per year (US list: $29,000, limited distribution) for RA
- Rinvoq — $30,400-$41,500 per year (with GoodRx coupon $5500/mo.) for AD and RA
- Dupixent — $29,000-$39,500 per year ($5300 month, limited distribution) for AD, subQ
CLICK HERE to access Cibinqo prescribing information https://cdn.pfizer.com/pfizercom/USPI_Med_Guide_CIBINQO_Abrocitinib_tablet.pdf
CLICK HERE to access the Pfizer press release for Cibinqohttps://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults