…..catching up on FDA Approvals
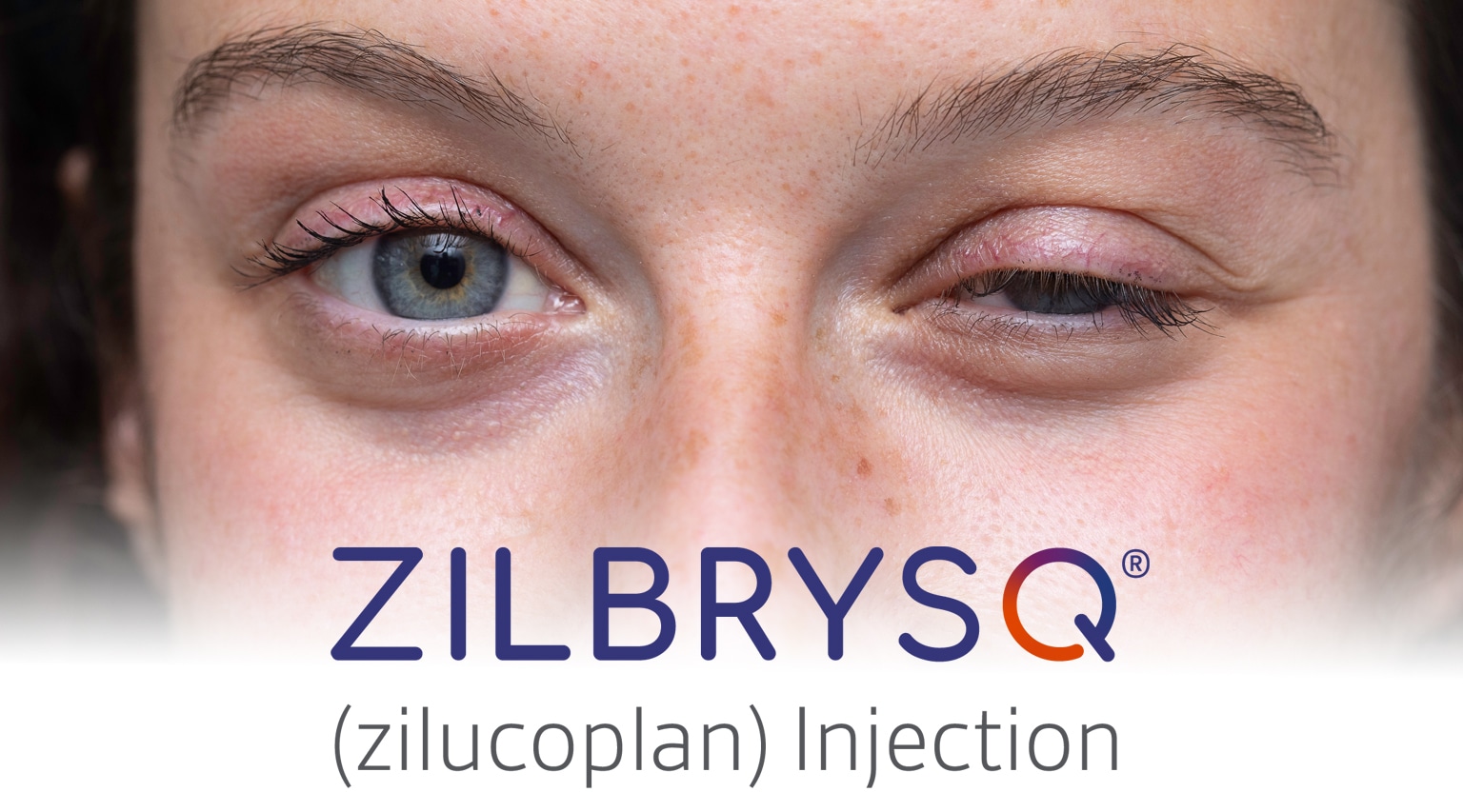
The FDA has approved a new subcutaneous therapy, Zilbrysq (zilucoplan) from UCB, for the treatment of adults with generalized myasthenia gravis. Zilbrysq is the first once-daily subcutaneous, targeted C5 complement inhibitor for gMG. It is the only once-daily gMG-target therapy for self-administration.
gMG is a rare autoimmune disease with a global prevalence of 100–350 cases per every 1 million people. Symptoms of the disease include severe muscular weakness, drooping eyelids, difficulty with swallowing, chewing and talking, as well as life-threatening weakness of the respiratory muscles.
Zilbrysq was approved with a Black Box Warning for serious meningococcal infections.
CLICK HERE to access prescribing information
UCB has not yet confirmed pricing for Zilbrysq.
Given that Zilbrysq is a self-administered, sub-q therapy with a Black Box Warning, it is expected that it will be channeled through specialty pharmacy limited distribution.