Last week the FDA approved a new infused therapy, Fyarro (sirolimus protein-bound particles for injectable suspension / albumin-bound) from Aadi Bioscience, for the treatment of adult patients with locally advanced unresectable or metastatic malignant perivascular epithelioid cell tumor (PEComa).
Fyarro is the first and only approved therapy for adults with this condition, an ultra-rare and aggressive form of sarcoma with a strong female predominance. Malignant PEComas may arise in almost any body site (typically the uterus, retroperitoneum, lung, kidney, liver, genitourinary, and gastrointestinal tract.
It is estimated that there are only about 100-300 new patients per year in the United States.
Fyarro’s wholesale acquisition cost was announced at $6,785.00 for a single-use 100 milligram vial. Utilization is dependent on the patient’s body mass and any dosing modification resulting in a cost of approximately $468,000 per year. Aadi said that “Discounts, such as Medicaid rebates, sales to 340B covered entities and VA facilities among others, will reduce the net price by approximately 15% to 20%.”
Given the small patient population in the US it is expected that Fyarro will be launched through a specialty pharmacy distribution (direct to physician) program.
Aadi Bioscience Announces FDA Approval of its First Product FYARRO for Patients with Locally Advanced Unresectable or Metastatic Malignant Perivascular Epithelioid Cell Tumor (PEComa)
LOS ANGELES, Nov. 23, 2021 — Aadi Bioscience, Inc., a biopharmaceutical company focusing on precision therapies for genetically-defined cancers with alterations in mTOR pathway genes, today announced that the U.S. Food and Drug Administration (FDA) has approved FYARRO™ (sirolimus protein-bound particles for injectable suspension) (albumin-bound) for intravenous use for the treatment of adult patients with locally advanced unresectable or metastatic malignant perivascular epithelioid cell tumor (PEComa). FYARRO is the first and only FDA-approved treatment for advanced malignant PEComa in adults.
Neil Desai, Ph.D., Founder, Chief Executive Officer and President of Aadi, stated, “We are thrilled to have received full FDA-approval of FYARRO. The approval of FYARRO is a momentous event not just for Aadi but, importantly for advanced malignant PEComa patients. We reiterate that all of us at Aadi are incredibly grateful to all of the people with advanced malignant PEComa, their families and caregivers, as well as the healthcare professionals who made the FYARRO clinical studies possible.”
“The approval of FYARRO, the first approved drug for advanced malignant PEComa, an aggressive sarcoma with a poor prognosis and few treatment options, will provide physicians with a new weapon for treating patients with this rare disease,” added Andrew Wagner, M.D., Ph.D., a senior oncologist at Dana-Farber Cancer Institute and the Principal Investigator in the pivotal AMPECT registrational trial. “In our AMPECT trial, FYARRO demonstrated durable responses in mTOR inhibitor-naïve patients with locally advanced unresectable or metastatic PEComa, with an acceptable and manageable safety profile. This is a drug that will be welcomed by the physician community as the only approved therapeutic option for patients with advanced malignant PEComa.”
In the Phase 2 registrational AMPECT trial the overall response rate as assessed by independent review was 39% (12/31) with 2 patients achieving a Complete Response after prolonged follow up. The median duration of response has not been reached with a median follow-up of 36 months, and a range of 5.6 to 55.5+ months and ongoing. Among responders, 92% had a response lasting greater than or equal to 6 months; 67% had a response lasting greater than or equal to 12 months; and 58% had a response lasting greater than or equal to 2 years. As is the case with other therapeutics of the mTOR class, the FYARRO prescribing information includes warnings and precautions related to stomatitis, myelosuppression, infections, hypokalemia, hyperglycemia, interstitial lung disease, hemorrhage, and hypersensitivity reactions. Grade 3 non-hematologic events occurring in more than 10% of patients included stomatitis, rash, fatigue and infections. Grade 3 laboratory abnormalities occurring in more than 10% of patients that worsened from baseline included lymphocytopenia, increased glucose, and decreased potassium. For detailed important safety information, please see below.
Brendan Delaney, Chief Operating Officer of Aadi, added, “We have built a strong commercial team and devised a thoughtful strategy in preparation for FYARRO’s launch. With FYARRO’s demonstrated clinical profile we believe it will become a standard of care for advanced malignant PEComa. We look forward to engaging with physicians to educate the market about this new treatment.”
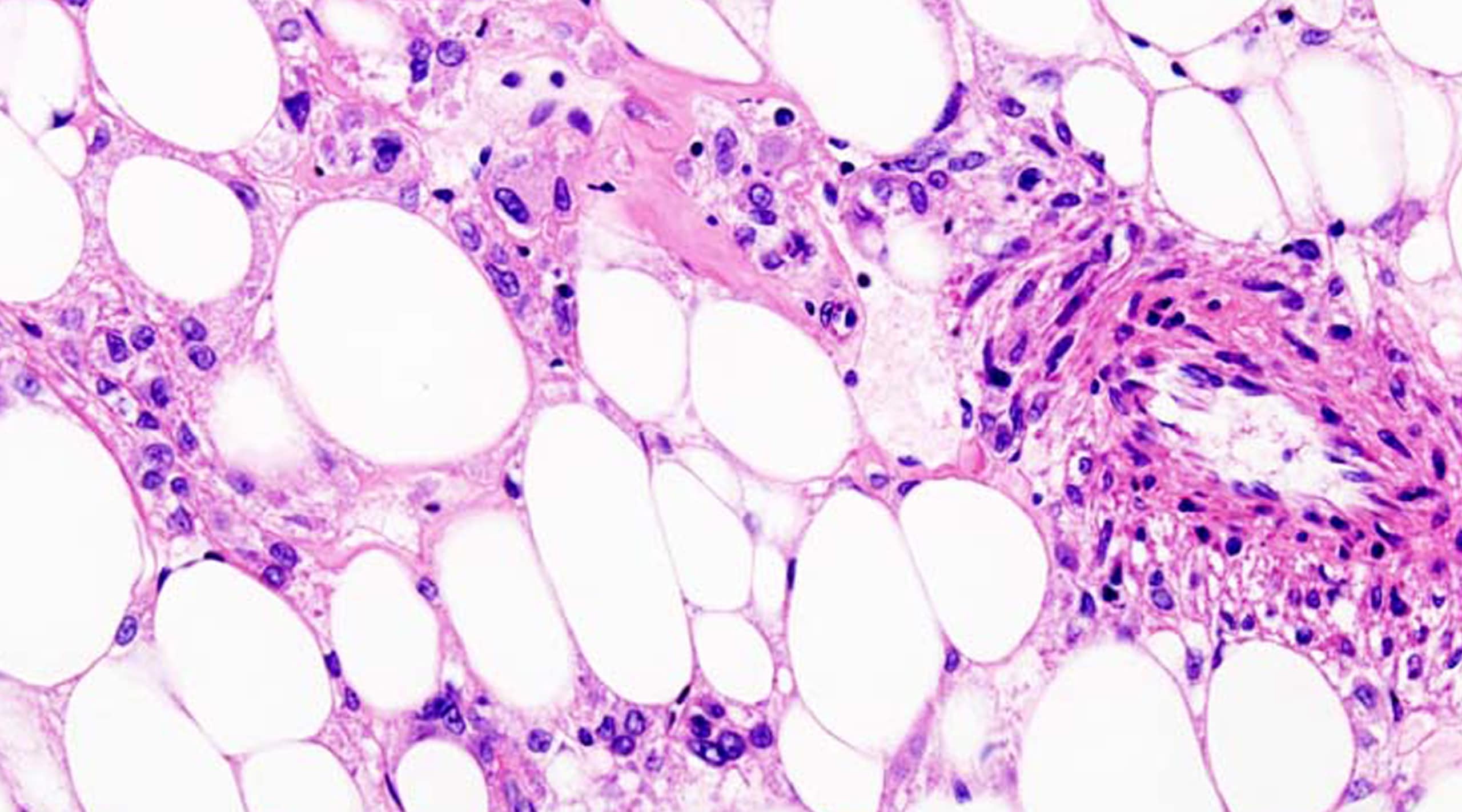
About Malignant PEComa
Advanced malignant PEComa, defined by the World Health Organization as ‘mesenchymal tumors composed of distinctive cells that show a focal association with blood-vessel walls and usually express both melanocytic and smooth muscle markers,’ are a rare subset of soft-tissue sarcomas, with an undefined cell of origin. While there is no formal epidemiology for malignant PEComa, with a female predominance) and can have an aggressive clinical course including distant metastases and ultimately death. The estimated prognosis based on retrospective reports is 12-16 months. Cytotoxic chemotherapies typically used for sarcoma show minimal benefit and there are currently no drugs approved for this disease. Malignant PEComas have been shown to frequently harbor mutations in the TSC1 and/or TSC2 genes that result in the activation of mTOR pathway making it a rational therapeutic target for this disease.