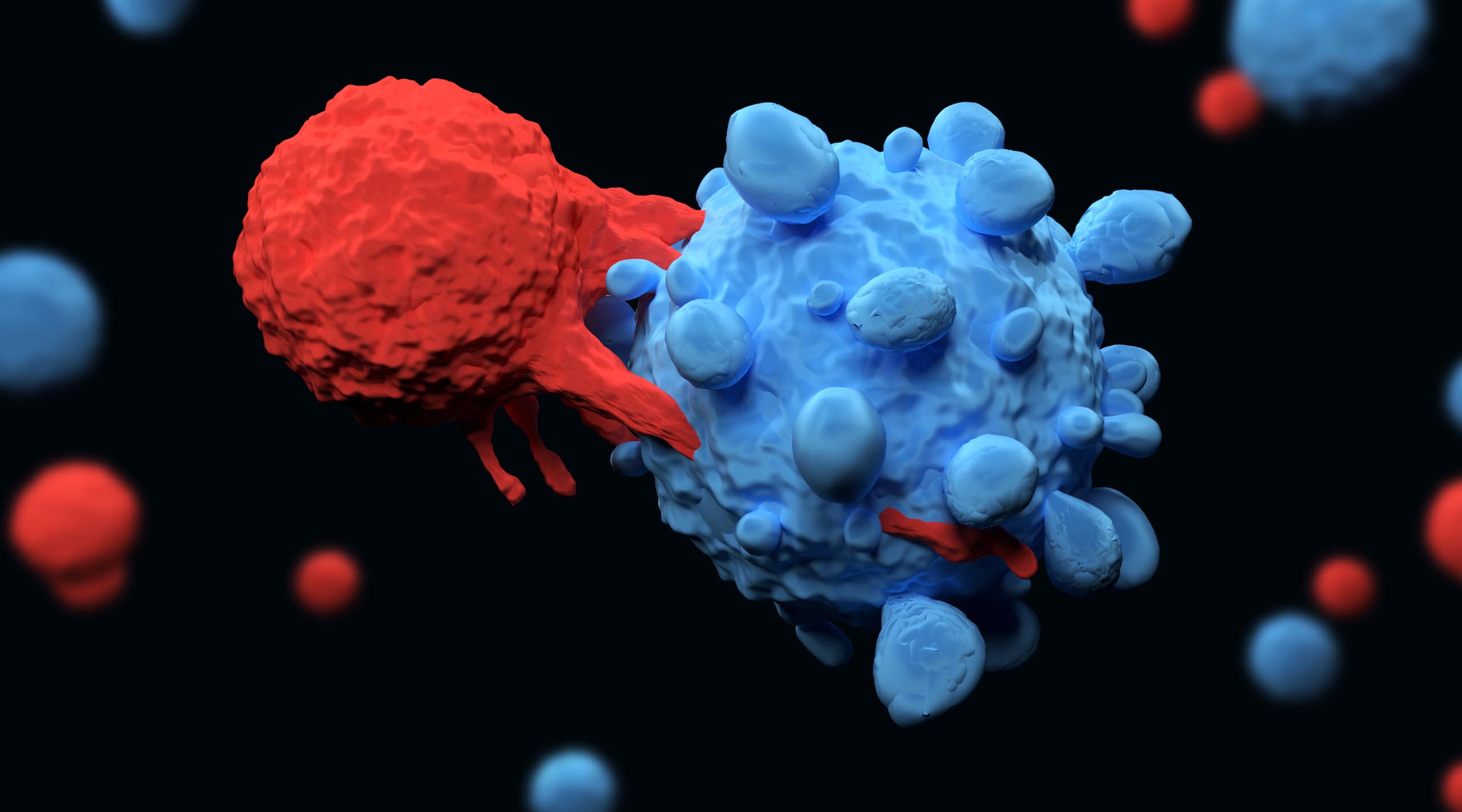
The FDA recently approved a new infused therapy, Carvykti (iltacabtagene autoleucel), from Janssen Biotech, for the treatment of adult patients with relapsed or refractory multiple myeloma. The therapy was developed in partnership with Legend Biotech. The partners will share marketing in the US.
Carvykti is a B-cell maturation antigen (BCMA)-directed genetically modified autologous chimeric antigen receptor (CAR) T-cell therapy. Each dose is customized using a patient’s own T-cells, which are collected and genetically modified, and infused back into the patient.
Carvykti will compete in the market with another BCMA-targeted CAR-T drug approved a year ago, Abecma from 2seventy bio. Janssen announced that Carvykti will launch at a wholesale acquisition cost of $465,000 vs. Abecma’s $419,500 list price.
Carvykti was approved with an extensive BOXED WARNING as well as a restrictive access REMS program.
Janssen did not announce details on distribution or whether access would be through a specialty pharmacy distribution program.