
Several Limited Distribution Deals Confirmed
Announcements for newly approved specialty drugs often state that the product will be available through specialty pharmacy in limited distribution. However, the press releases rarely

Announcements for newly approved specialty drugs often state that the product will be available through specialty pharmacy in limited distribution. However, the press releases rarely
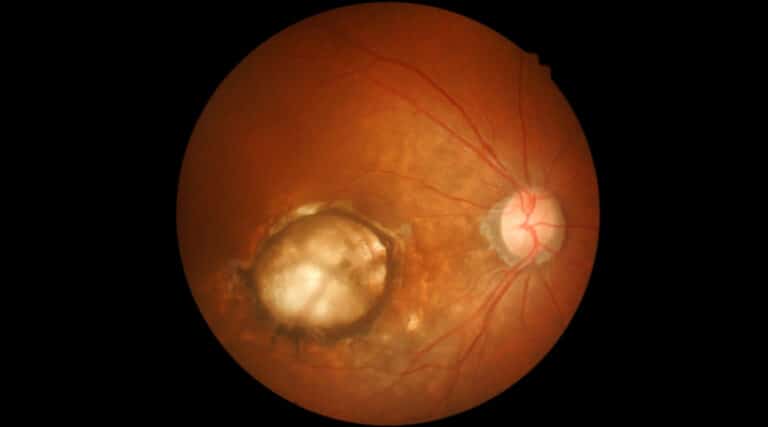
The FDA approved a new therapy, Susvimo (ranibizumab injection) from Genentech, indicated for intravitreal use via ocular implant for wet age-related macular degeneration (AMD) with

A little over two years ago we reported on the approval of yet another biosimilar to Humira (yawn), Cyltezo (adalimumab-adbm) a sub-q therapy from Boehringer

Last week we published a review of the 2021 Medication Access Report which detailed a variety of access barriers including COVID-19, logistics, financial burdens, and

We’ve written frequently on two of the biggest trends in channel access, the shift away from buy-and-bill to other, less costly, sites of service as

We all know that the past year has been nothing short of a massive monkey wrench thrown into the US healthcare machinery and the impacts

The debate over rebate appropriateness has heated up over the past year. The focus on drug spend is now burning bright, especially given the current

Earlier this month the FDA approved a new Infused therapy, Tivdak (tisotumab vedotin-tftv) from Seagen Inc., for adult patients with recurrent or metastatic cervical cancer
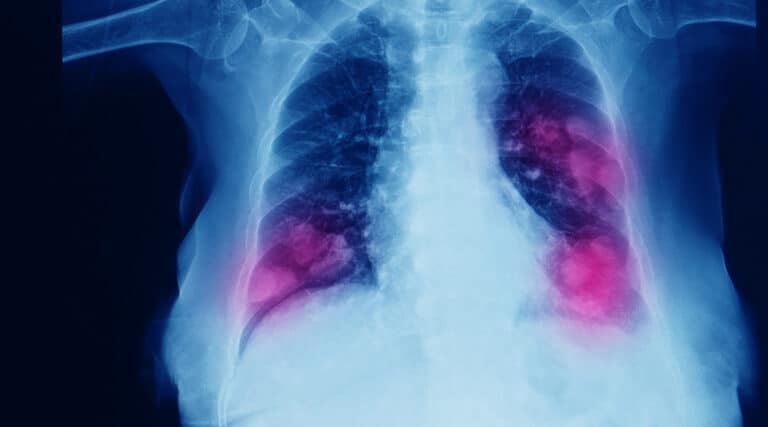
Earlier this month the FDA approved a new ORAL specialty therapy, Exkivity (mobocertinib) from Takeda Pharmaceutical, for the treatment of adult patients with advanced or

Frequent readers of this report know that one of our favorite topics is health-system-owned specialty pharmacies. So, it was with great interest that we read
We use cookies to improve your experience on our site. By using our site, you consent to cookies.
Manage your cookie preferences below:
Essential cookies enable basic functions and are necessary for the proper function of the website.
Statistics cookies collect information anonymously. This information helps us understand how visitors use our website.
Marketing cookies are used to follow visitors to websites. The intention is to show ads that are relevant and engaging to the individual user.