
Hy-Vee Launches Telemarketing Subsidiary
We are always on the hunt for stories and events that can be impactful on the specialty pharmacy segment. The story below recently crossed out

We are always on the hunt for stories and events that can be impactful on the specialty pharmacy segment. The story below recently crossed out

Announcements for newly approved specialty drugs often state that the product will be available through specialty pharmacy in limited distribution. However, the press releases rarely
The FDA just approved not ONE, but TWO therapies in the already crowded immunology category. The two include a new NDA for Cibinqo (abrocitinib) from Pfizer and an expanded indication for
At the end of December, the FDA approved yet another biosimilar, Yusimry (adalimumab-aqvh) from Coherus Bioscience. Yusimry is a tumor necrosis factor blocker approved as

Last week the FDA approved a new ORAL therapy, Recorlev (levoketoconazole) from Xeris Biopharma Holdings, for the treatment of endogenous hypercortisolemia in adult patients with
We are catching up on the wave of FDA approvals that happen in the waning hours of the year. The FDA approved Apretude (cabotegravir), from

In the final hours of 2021, the FDA approved a number of new drugs. One of these therapies is Adbry (tralokinumab-ldrm) from Leo Pharma. Adbry,
Last week the FDA approved Leqvio, from Novartis Pharmaceuticals. Leqvio is a first-in-class, RNAi sub-Q therapy indicated as an adjunct to diet and maximally tolerated

The public relations battle surrounding White Bagging is heating up. Who’s on each side? Depends on who you ask….! On one side, hospitals are claiming that
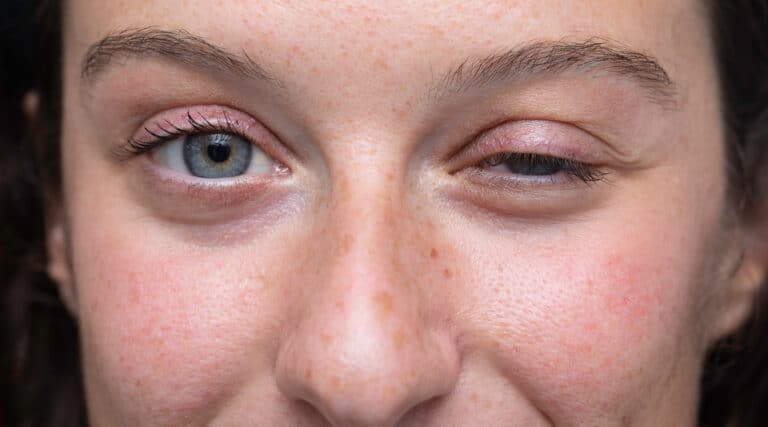
The FDA has approved a new INFUSED biologic, Vyvgart (efgartigimod alfa-fcab, Argenx), for treatment of generalized Myasthenia Gravis (gMG) in adults who are acetylcholine receptor
We use cookies to improve your experience on our site. By using our site, you consent to cookies.
Manage your cookie preferences below:
Essential cookies enable basic functions and are necessary for the proper function of the website.
Statistics cookies collect information anonymously. This information helps us understand how visitors use our website.
Marketing cookies are used to follow visitors to websites. The intention is to show ads that are relevant and engaging to the individual user.
You can find more information about our Cookie Policy and Privacy Policy.