
Option Care Acquires SPNN
Option Care Health announced that it has acquired the Specialty Pharmacy Nursing Network (SPNN) for $60 million in an all-cash transaction. SPNN will maintain its

Option Care Health announced that it has acquired the Specialty Pharmacy Nursing Network (SPNN) for $60 million in an all-cash transaction. SPNN will maintain its
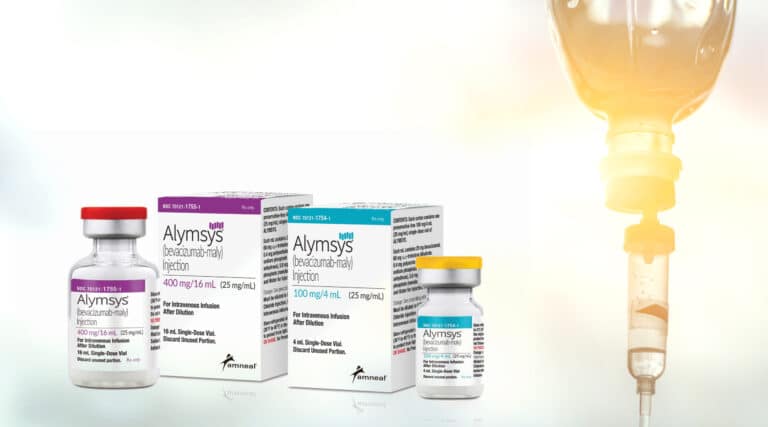
The FDA approved the 35th biosimilar last week, Alymsys (bevacizumab-maly) from Amneal Pharmaceuticals, for seven cancer indications of the reference product, Avastin from Genentech. It

The National Home Infusion Association (NHIA) recently published the results of a survey of patients who had received a home chemotherapy infusion to gauge their

Last week the FDA approved a new ORAL specialty therapy, Vijoice (alpelisib) from Novartis Pharmaceuticals, indicated for breast cancer patients with evidence of mutation in
The FDA recently approved Opdualag (nivolumab and relatlimab-rmbw) from Bristol Myers Squibb, a first-in-class, fixed-dose dual immunotherapy combination treatment of the PD-1 inhibitor nivolumab and

HIPAA…. the lurking threat to every specialty pharmacy. That’s a provocative statement….. but one that rings all too true. Each year we hear of yet

The FDA recently approved a new oral suspension therapy, Ztalmy (ganaxolone) to treat seizures associated with cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder (CDD) in patients
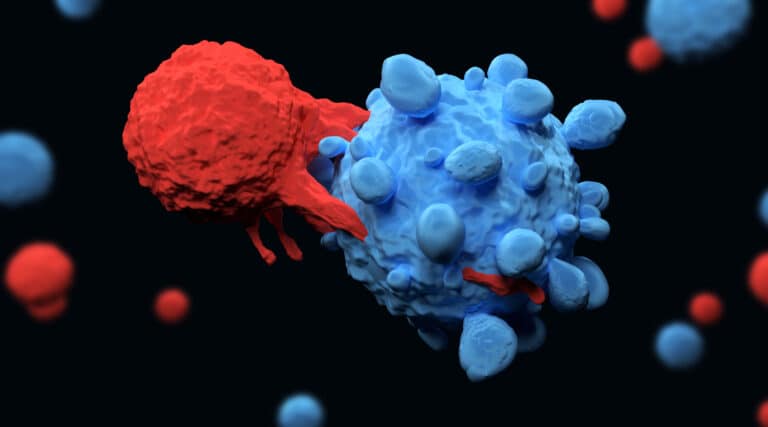
The FDA recently approved a new infused therapy, Carvykti (iltacabtagene autoleucel), from Janssen Biotech, for the treatment of adult patients with relapsed or refractory multiple
Announcements for newly approved specialty drugs often state that the product will be available through specialty pharmacy in limited distribution. However, the press releases rarely

Over the past decade more and more specialty pharmacies obtained wholesaler/distributor (WDD / 3pl) licenses in addition to the traditional pharmacy license. Most of the therapies
We use cookies to improve your experience on our site. By using our site, you consent to cookies.
Manage your cookie preferences below:
Essential cookies enable basic functions and are necessary for the proper function of the website.
Statistics cookies collect information anonymously. This information helps us understand how visitors use our website.
Marketing cookies are used to follow visitors to websites. The intention is to show ads that are relevant and engaging to the individual user.