Home Infusion Gaining Traction in Marketplace
Back in February we sent a report detailing an innovative network approach to alternate site / home infusion and it seems to be a topic

Back in February we sent a report detailing an innovative network approach to alternate site / home infusion and it seems to be a topic

The FDA this week issued final guidance related to the importation of drugs from Canada. Importation was passed late last year but many questions remained unresolved.

A significant number of specialty pharmacies have contracted as a 340b dispensing pharmacy often for multiple ‘program eligible’ hospitals. As most know, manufacturers have recently

Earlier this month the FDA approved an ORAL specialty therapy, Cuvrior (trientine tetrahydrochloride) from Orphalan SA, for the treatment of adults with stable Wilson’s disease

Announcements for newly approved specialty drugs often state that the product will be available through specialty pharmacy via limited distribution (LD). However, the press releases

We’ve written about the emergence of telehealth services for the past several years. As then, we continue to see positive uptake, albeit much slower than

The rumors about a big divestiture move by Centene Corp. finally materialized last week. Centene announced that they are spinning off both its PantherRx Rare
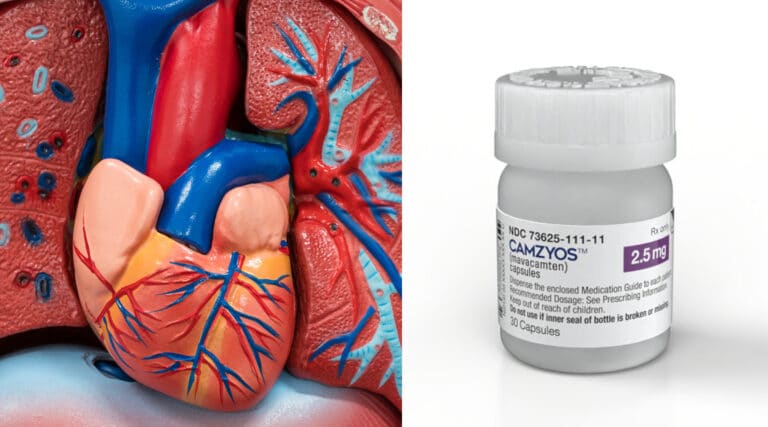
Over the years few new specialty therapies have been approved for Cardiology conditions. That may be about to finally change with a recent FDA approval.

We’ve written frequently on two of the biggest trends in channel access, the shift away from buy-and-bill to other, less costly, sites of service as

Limited distribution (LD) has contributed to the growth of a couple dozen specialty pharmacies over the past decade. When a pharmaceutical manufacturer selects a specialty pharmacy
We use cookies to improve your experience on our site. By using our site, you consent to cookies.
Manage your cookie preferences below:
Essential cookies enable basic functions and are necessary for the proper function of the website.
Statistics cookies collect information anonymously. This information helps us understand how visitors use our website.
Marketing cookies are used to follow visitors to websites. The intention is to show ads that are relevant and engaging to the individual user.