
FDA Approves the 9th Humira Biosim – Yuflyma
The excitement in the air is palpable…. Yes, another biosimilar to Humira has been approved by the FDA!! Hallelujah!! Ummm….. wait….. the degree of excitement may be….. exaggerated.

The excitement in the air is palpable…. Yes, another biosimilar to Humira has been approved by the FDA!! Hallelujah!! Ummm….. wait….. the degree of excitement may be….. exaggerated.

A few weeks ago, we sent a Report asking whether value-based (VB) contracting had a heartbeat. Today we have evidence that a meaningful heartbeat has been

From time to time we run across an article that offers more than just recent industry news. Today we stumbled across an article that we suggest

Announcements for newly approved specialty drugs often state that the product will be available through specialty pharmacy in limited distribution. However, the press releases rarely
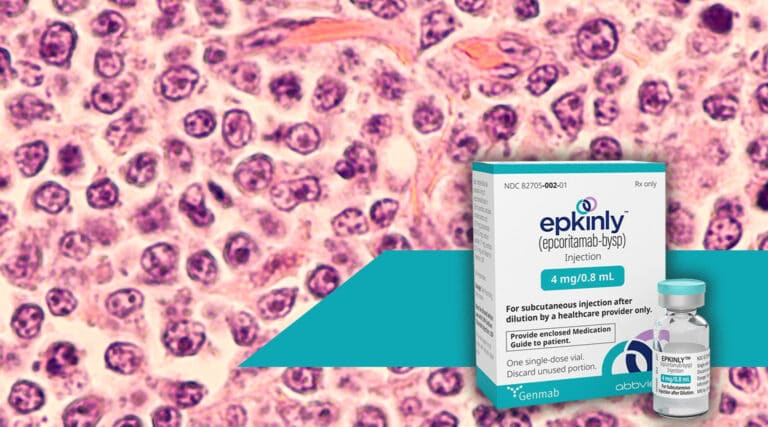
The FDA recently approved a new subcutaneous therapy, Epkinly (epcoritamab-bysp) from Genmab, for the treatment of adult patients with relapsed or refractory (R/R) diffuse large

The FDA just approved a new rare, GENE therapy, Vyjuvek (beremagene geperpavec-svdt) from Krystal Biotech, indicated for the treatment of patients six months of age

A survey just released by the Pharmaceutical Strategies Group confirmed what we already know….. that the COST of specialty medications is the tipppty toppest key concern of
The hottest topic in the media these days is Artificial Intelligence (AI). It seems like everyone is talking about it. But that may be short sighted if

The FDA recently approved a new subcutaneous specialty therapy, Uzedy (risperidone) from TEVA and MedinCell, an atypical antipsychotic indicated for the treatment of schizophrenia in

We’ve reported several times on the disparity of specialty drug pricing based on where the drugs are administered. None of our readers would be surprised that
We use cookies to improve your experience on our site. By using our site, you consent to cookies.
Manage your cookie preferences below:
Essential cookies enable basic functions and are necessary for the proper function of the website.
Statistics cookies collect information anonymously. This information helps us understand how visitors use our website.
Marketing cookies are used to follow visitors to websites. The intention is to show ads that are relevant and engaging to the individual user.
You can find more information about our Cookie Policy and Privacy Policy.